Funding
|
NSF CHE - 1956302 (Chemical Catalysis)
Title: Development of Catalysts and Ligands for Alkyne Metathesis NSF Award Abstract link LSU Reveille News link LSU Chemistry News link |
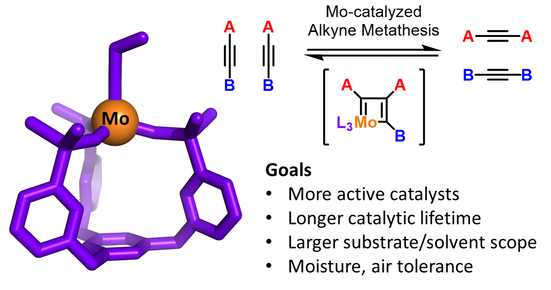
Alkyne Metathesis Catalyst Development
Alkyne metathesis is a reaction that scrambles alkynes (triple bonds) within a reaction. It is an extremely useful technique for synthesizing macrocycles and cage-molecules with well-defined structures and pores. However, the current state-of-the-art catalysts are far from perfect. Since they are using high-valent metals such as Mo(VI) and W(VI), they are very sensitive to water and oxygen, and has very limited substrates scopes. Our goal is to investigate the fundamental design rules and ligand effects in Mo,W-based catalysts to develop the next generation alkyne metathesis catalysts.
Alkyne metathesis is a reaction that scrambles alkynes (triple bonds) within a reaction. It is an extremely useful technique for synthesizing macrocycles and cage-molecules with well-defined structures and pores. However, the current state-of-the-art catalysts are far from perfect. Since they are using high-valent metals such as Mo(VI) and W(VI), they are very sensitive to water and oxygen, and has very limited substrates scopes. Our goal is to investigate the fundamental design rules and ligand effects in Mo,W-based catalysts to develop the next generation alkyne metathesis catalysts.
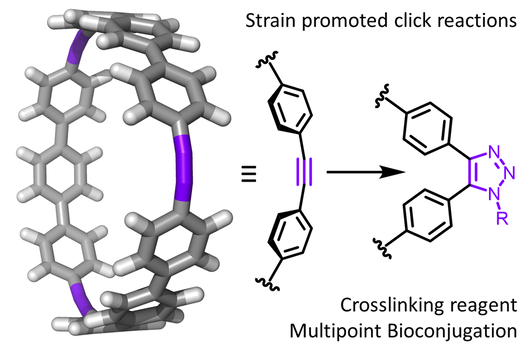
Strained Carbon Nanohoops
Carbon nanohoops are fully conjugated with para-linked phenylenes and alkynes. Because the phenylenes and alkynes are forced into a curvature, they are extremely strained and very reactive. We are preparing these nanohoops in an efficient manner using alkyne metathesis and investigating their strain-release reactivities. We envision that these reaction can be used in cross-linking agents in polymer and bioconjugation methods in chemical biology.
Carbon nanohoops are fully conjugated with para-linked phenylenes and alkynes. Because the phenylenes and alkynes are forced into a curvature, they are extremely strained and very reactive. We are preparing these nanohoops in an efficient manner using alkyne metathesis and investigating their strain-release reactivities. We envision that these reaction can be used in cross-linking agents in polymer and bioconjugation methods in chemical biology.
Lithium-Ion Extraction and Separation
Lithium is a crucial element for the modern world as they are used for lithium-ion batteries to power electric vehicles and portable electronic devices. Due to the increasing demand of lithium and a sustainable closed-loop recycling system, efficient and cost-effective lithium extraction techniques are being investigated. We are developing various organic molecules that can effectively selectively bind lithium against other metal ions. The goal is to extract and separate lithium-ion (or salts) from mineral brine, sea water, or postconsumer batteries ores.
Lithium is a crucial element for the modern world as they are used for lithium-ion batteries to power electric vehicles and portable electronic devices. Due to the increasing demand of lithium and a sustainable closed-loop recycling system, efficient and cost-effective lithium extraction techniques are being investigated. We are developing various organic molecules that can effectively selectively bind lithium against other metal ions. The goal is to extract and separate lithium-ion (or salts) from mineral brine, sea water, or postconsumer batteries ores.
Equipment